Ren LY, Hong ZN, Liu ZD, Xu RK. ATR–FTIR investigation of mechanisms of Bacillus subtilis adhesion onto variable- and constant-charge soil colloids. Colloids and Surfaces B: Biointerfaces, doi: 10.1016/j.colsurfb.2017.11.067
Abstract
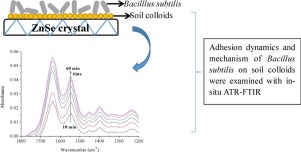
The primary objective of this study was to determine the capacity for and the mechanisms of adhesion of Bacillus subtilis onto variable- and constant-charge soil colloids. The adhesion process was investigated using in situ attenuated total reflectance (ATR)–Fourier transform infrared spectroscopy (FTIR), zeta potential, and batch adhesion experiments. The maximum adhesion capacity of B. subtilis on the colloids of Oxisol, Ultisol, and Alfisol reached 699.17, 462.56, and 258.82 mg g−1, respectively. B. subtilis adhesion to all three soil colloids decreased as the suspension pH increased from 3 to 8. Saturation coverage and adhesion rate constant values were calculated with the pseudo-first-order kinetics equation using the absorbance at 1458 cm−1. Both values were highest for Oxisol, followed by Ultisol, and lowest for Alfisol. These observations are consistent with the surface charges of these soil colloids. A larger positive charge on variable-charge soils (Oxisol and Ultisol) increased B. subtilis adhesion relative to that of constant-charge soil (Alfisol). This is in agreement with the interaction energy between B. subtilis and soil colloids, which was calculated using the Derjaguin–Landau–Verwey–Overbeek theory. As revealed by ATR–FTIR spectroscopy, chemical bonds formed by protein, phosphate, and COOH groups on B. subtilis, as well as iron and aluminum hydroxyl groups in soil, contributed to B. subtilis adhesion to soil colloids. Therefore, chemical bond formation and electrostatic interaction are two major mechanisms of B. subtilis adhesion onto soil colloids.